Extreme recycling for a better tomorrow.
Title: Investigating Electrolytic Screens in Gas Separation: A Novel Approach to Efficient Hydrogen Production
Abstract
This paper explores the viability of using stacked screen electrodes for the electrolytic decomposition of water and urea-containing liquids (such as urine). Rather than traditional rod or plate electrodes, this method uses alternating polarity metal screens arranged vertically. The setup is intended to allow for efficient gas evolution and natural bubble migration upward through the stack, potentially simplifying gas harvesting and reducing ohmic losses. We evaluate the physical principles, engineering feasibility, potential gas separation behavior, and the implications for both mobile and stationary energy production systems.
1. Introduction
Conventional electrolysis systems rely on solid electrodes—typically plates or rods—to apply potential across a fluid and split water or urea into useful gases. This paper considers a nontraditional architecture: a vertical stack of alternating charged conductive screens, separated by dielectric spacers. This setup could potentially enhance bubble migration, reduce electrode fouling, and simplify maintenance and gas separation. The goal is to develop a low-cost, easily serviceable system capable of processing human waste streams (notably urine) or water for hydrogen-rich gas production.
2. Theoretical Framework and Electrochemical Basis
Electrolysis of water (H₂O) and urea [(NH₂)₂CO] follows well-known pathways:
Water Electrolysis: Produces hydrogen and oxygen.
Urea Electrolysis: Produces hydrogen, nitrogen, and sometimes ammonia, at a lower cell voltage (~0.37–0.6 V per cell).
Electrolytic reactions benefit from high surface area and close inter-electrode distances, both of which can be achieved using conductive mesh or screen materials. The stacked screen approach can increase effective reaction surface area without significantly increasing the footprint of the device.
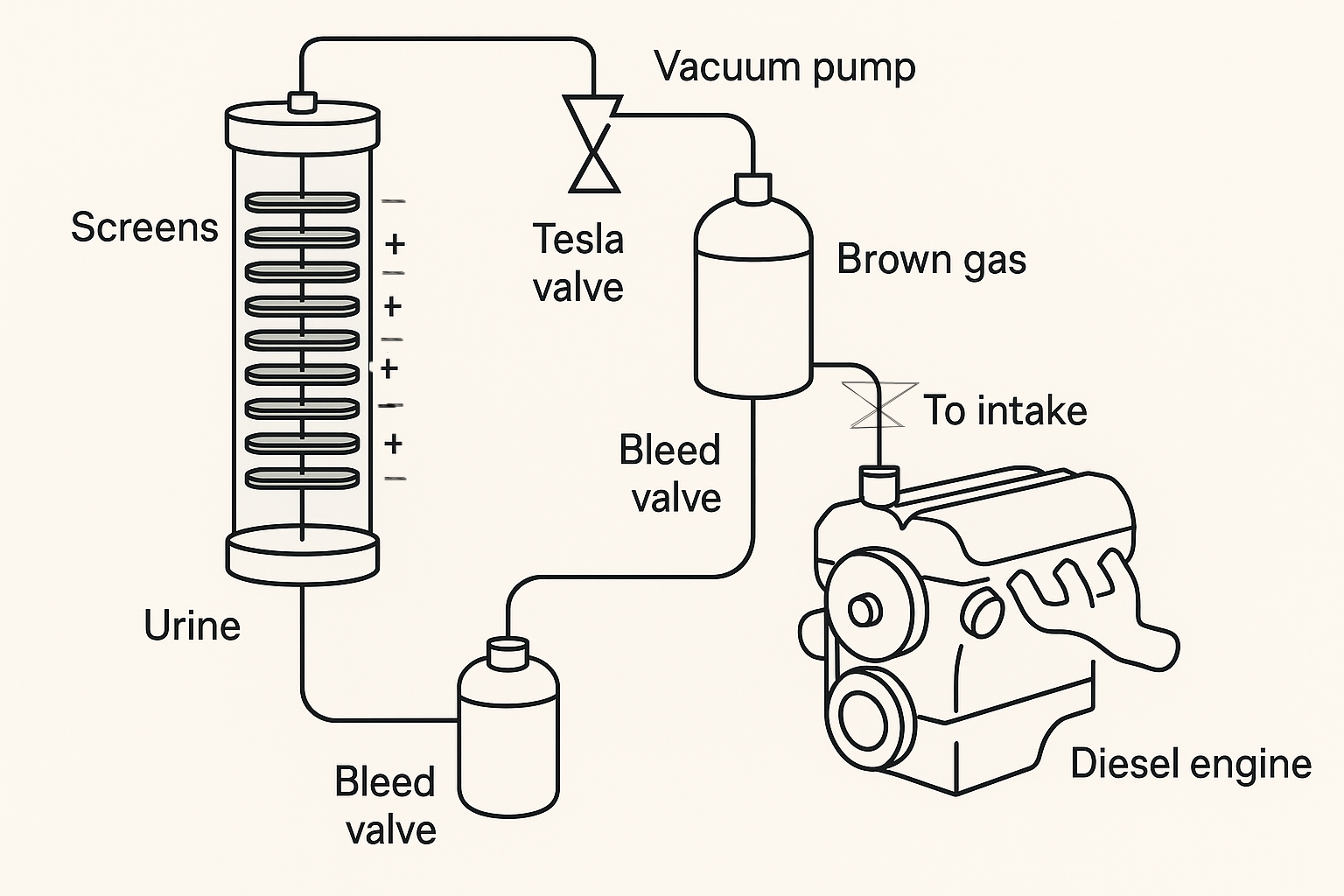
3. Screen Stack Configuration and Gas Behavior
3.1. Electrode Design
Stainless steel or nickel wire mesh
Alternating polarity with dielectric spacers (e.g., PTFE washers)
Configured vertically inside a sealed tube
3.2. Bubble Transport and Separation
As electrolysis proceeds, hydrogen and oxygen (or nitrogen) bubbles form on screen surfaces and naturally rise. This vertical alignment allows for passive separation by buoyancy:
Light hydrogen bubbles rise faster, collecting at the top
Heavier oxygen or nitrogen may be drawn off from different zones or mixed depending on need
3.3. System Architecture
Liquid Chamber: Filled with filtered urine or water
Screen Stack: Alternating polarity, powered by DC source
Gas Outlet: Positioned at the top with a vacuum pump or pressure differential to draw out mixed gas
Tesla Valve or Check Valve: Prevents backflow into the liquid chamber
4. Efficiency Considerations and Applications
This configuration may offer benefits such as:
Reduced energy input due to urea’s lower electrolysis voltage
Simpler gas extraction (natural separation by density)
Modular and scalable design for small systems (e.g., RVs, farms, disaster relief)
While mixed gas is not ideal for storage, immediate on-demand burning (e.g., in a combustion engine or torch) can avoid the need for gas purification.
5. Implementation Challenges
Corrosion Resistance: Mesh must withstand electrochemical wear
Gas Mixing: Without precise separation, combustion must handle variable gas content
Foaming: Urea-containing solutions can foam; surfactant management may be required
6. Future Research Directions
CFD modeling of bubble rise and separation dynamics
Lab-scale prototype testing with urine and water
Integration with biodiesel or hydrogen-injection hybrid systems
Conclusion
Screen-based electrolysis offers a compelling alternative to traditional electrode geometries, particularly when working with biologically derived fluids like urine. Its simplicity, potential low cost, and natural gas separation behavior make it promising for off-grid or sustainable energy systems.
References
Boggs, B. K., King, R. L., & Killeen, G. R. (2009). Urea electrolysis: direct hydrogen production from urine. Chemical Communications, (32), 4859–4861.
Rozendal, R. A., et al. (2006). Efficiency of hydrogen production in microbial electrolysis cells. Environmental Science & Technology, 40(17), 5181–5186.
Davis, J., et al. (2013). Electrochemical conversion of human waste: a microbial fuel cell case study. Bioresource Technology, 150, 281–285.
Tesla, N. (1920). Valve. U.S. Patent No. 1,329,559.
Pletcher, D., & Walsh, F. C. (1990). Industrial Electrochemistry. Springer.
Logan, B. E., & Regan, J. M. (2006). Electricity-producing bacterial communities in microbial fuel cells. Trends in Microbiology, 14(12), 512–518.